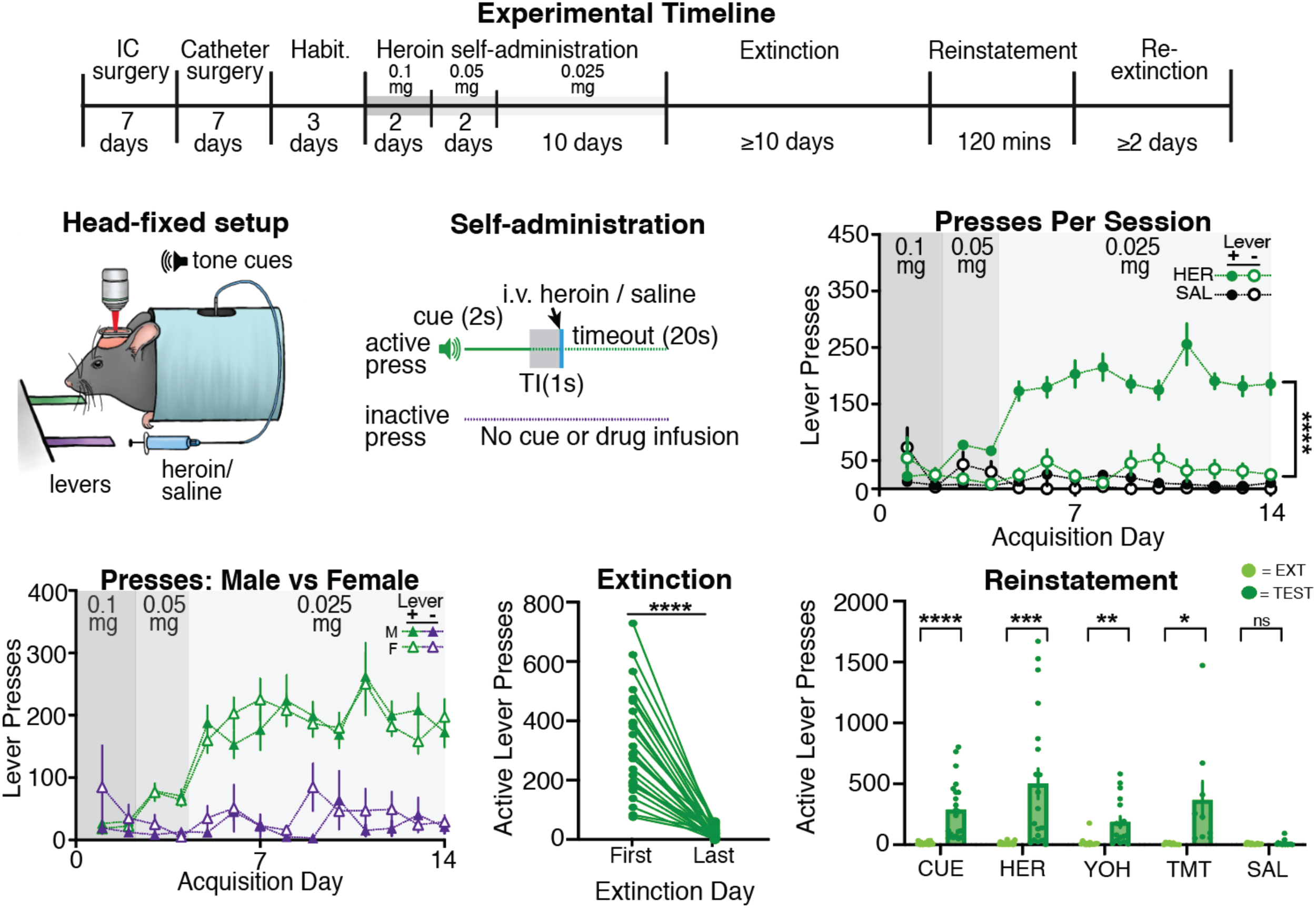
Head-restrained self-administration
The Otis Lab employs a novel behavioral assay that allows for simultaneous head-restrained self-administration and multiphoton imaging to better characterize and manipulate the neuronal circuit activity during operant reward conditioning. Similar to freely moving drug self-administration studies, our head-restrained procedure allows mice to acquire, extinguish, and reinstate drug-seeking behaviors as well as monitor neural dynamics from the onset of drug use through relapse.
Dr. Otis designed this new approach for drug self-administration that not only uniquely targets limitations in historical applications of multiphoton microscopy, but also builds upon the foundational research provided by freely moving drug self-administration studies. For examples of head-restrained behavioral experiments, check out Vollmer et al 2021 Frontiers in Behavioral Neuroscience, Grant et al 2021 eLife, Vollmer et al 2022 Nature Communications, or Paniccia et al 2024 Neuron.
Two-photon microscopy
The Otis Lab has two, state-of-the-art, multiphoton microscopes. The first is a Bruker system that allows for imaging in awake, behaving mice. This microscope is equipped with lasers that allow simultaneous calcium imaging and optogenetics or uncaging, with a spatial light modulator that allows single-cell stimulation or inhibition within each field of view. In addition, this microscope has been equipped with LEDs for full field optogenetics during imaging. The second system is a custom-built two-photon microscope that can be used for both for imaging in vivo or ex vivo.
Dr. Otis was among the first ever to record the activity of axons deep in the brain, and also to optogenetically manipulate these axons while recording from downstream neurons. The lab plans to continue such approaches to better understand the neural circuit mechanisms of reward seeking and drug addiction. For an example of two-photon imaging experiments, check out Otis et al 2017 Nature, Otis et al 2019 Neuron, Grant et al 2021 eLife, Vollmer et al 2022 Nature Communications, or Paniccia et al 2024 Neuron.
Patch-clamp electrophysiology
The Otis Lab has a state-of-the art patch-clamp electrophysiology system, equipped with components that allow optogenetics or two-photon imaging ex vivo. This system can be used to study the synaptic architecture of neural circuits. In addition, we use patch-clamp electrophysiology to study how neural circuits change in response to experience. Such plasticity is a major component of neuropsychiatric diseases, and therefore is a major focus of the lab. For examples of patch-clamp electrophysiology experiments, check out Otis et al 2017 Neuropsychopharmacology, Otis et al 2017 Nature, Otis et al 2018 Frontiers in Behavioral Neuroscience, Otis et al 2019 Neuron, Vollmer et al 2022 Nature Communications or Paniccia et al 2024 Neuron.
Circuit mapping
The Otis lab uses a combination of viral strategies to identify the precise connectivity of neural circuits. These strategies include, but are not limited to, cre-driven rabies tracing, retrograde toxin labeling, anterograde tracing, as well as a combination of the above techniques that allow us to identify the precise architecture of neural circuits. For an example of such strategies, see the extended figures from Otis et al 2017 Nature, Vollmer et al 2022 Nature Communications or Paniccia et al 2024 Neuron.
Behavioral Chemogenetics and Optogenetics
In addition to using optogenetics during two-photon imaging and patch-clamp electrophysiology experiments, we using optogenetics and chemogenetics to determine the neural mechanisms that underly the behaviors of freely moving animals. As such, we couple the approaches with a variety of custom-designed behavioral assays, such as Pavlovian conditioning, operant behaviors, conditioned place preference, and more. For examples of these behaviors, check out Otis et al 2017 Nature, Otis et al 2019 Neuron, Vollmer et al 2022 Nature Communications, or Paniccia et al 2024 Neuron.